Market Access & Health Technology Assesment: Japan
Market Access & Health Technology Assesment: Japan is a must-have asset for any company operating in Brazil or looking to enter the market.
Prepared in association with Nishimura & Asahi, a leading Brazilian law firm.
March 2025
1. Post Market-Approval Processes and Regulations: Japan
- What are the pricing models, processes and principles for originator drugs?
Drugs are provided by a medical institution or pharmacy to a patient based on the NHI Drug Price (a patient pays 10%, 20% or 30% (depending on age and other conditions) of the NHI Drug Price).
As detailed in Question I. 2. a. above, after a marketing authorization of a new drug is granted, the marketing authorization holder submits an application to the MHLW for listing the new drug on the NHI Drug Price Standard List, and the Central Social Insurance Medical Council determines the NHI Drug Price.
The NHI Drug Price of a new originator drug is calculated, if there is a similar drug, by (1) the comparable drug method, and if there is no similar drug, by (2) the cost accounting method.
Under the comparable drug method ((1) above), first, an existing drug that has a similar effect to the new originator drug is investigated and identified. Then, the NHI Drug Price of the new originator drug is calculated so that there is no significant price difference between the new originator drug and the similar drug. If the new originator drug is found to be more useful than the similar drug (e.g., having superior efficacy and/or safety), a certain amount of price is added.
Under the cost accounting method ((2) above), the NHI Drug Price of a new originator drug is calculated based on raw material cost and manufacturing cost. The projected amount of operating income and other amounts will be added based on the usefulness of the new originator drug compared to existing treatments.
After the NHI Drug Price is calculated either by the comparable drug method or the cost accounting method, a foreign average price adjustment will be made if there is a gap between the calculated NHI Drug Price and the average sales prices in foreign countries. More details are provided in Question IV. 2. below.
[Overview of NHI Drug Price Standards of originator drugs]

Both (i) the sales price from a marketing authorization holder to a pharmaceutical wholesaler and (ii) the sales price from the pharmaceutical wholesaler to a medical institution or pharmacy are freely determined by an agreement between the parties through free competition.
Over-the-counter drugs are not covered by the NHI, and therefore the prices at which they are provided to patients are freely determined.
As detailed in Question XIII. 2. below, while vaccines are not covered by the NHI, in many cases, medical institutions provide vaccines to citizens as “routine vaccination” on consignment from the local government. Therefore, medical institutions purchase vaccines from pharmaceutical wholesalers at the prices determined by an agreement between national, prefectural, or municipal governments and marketing authorization holders or pharmaceutical wholesalers. The price of vaccination by medical institutions is determined by agreement between the local government and the medical institution.
2 . What are the pricing models, processes and principles for generics and biosimilars drugs?
With regard to generics and biosimilar drugs, a marketing authorization holder also submits an NHI Drug Price application after a marketing authorization is granted as stated in Question I. 2. a. However, unlike originator drugs, there is no negotiation process between the marketing authorization holder and the MHLW, nor the consultation process with Central Social Insurance Medical Council in the determination of NHI Drug Price of generics and biosimilar drugs.
The methods to calculate the NHI Drug Price of generic and biosimilar drugs are different from those for originator drugs.
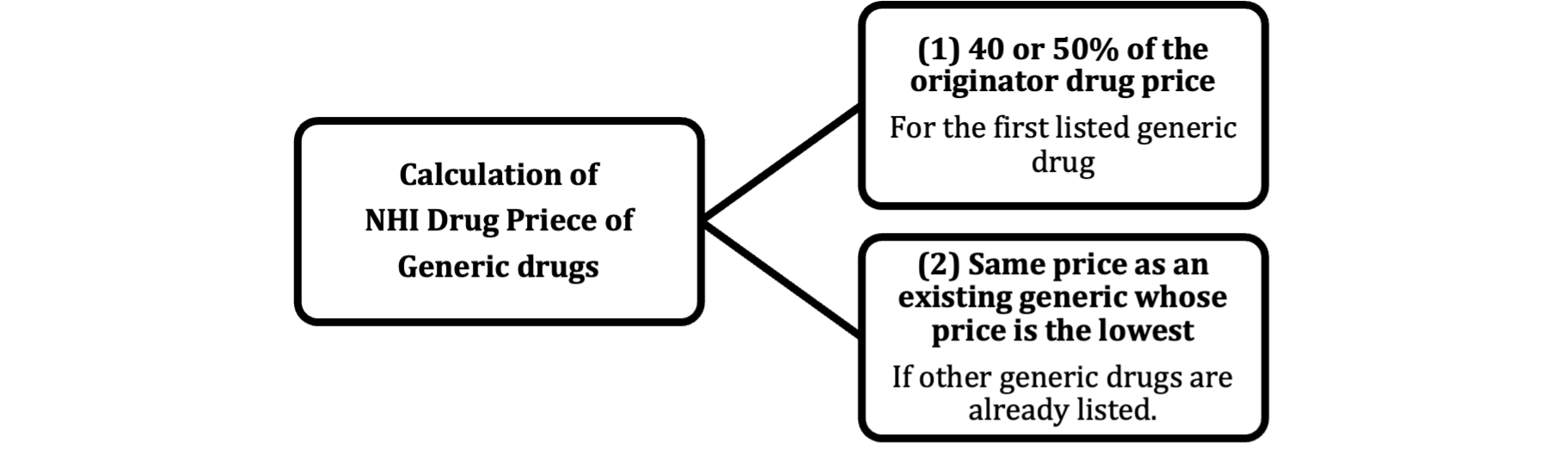
The NHI Drug Price of the first listed generic drug is 50% of the price of the originator drug calculated by the comparable drug method; however, if (i) it is an oral drug and (ii) there are more than 7 listed generic drugs, the NHI Drug Price will be 40%. If there are generic drugs that are listed earlier, the NHI Drug Price of an additional generic will be the same as the NHI Drug Price of an existing generic whose price is the lowest.
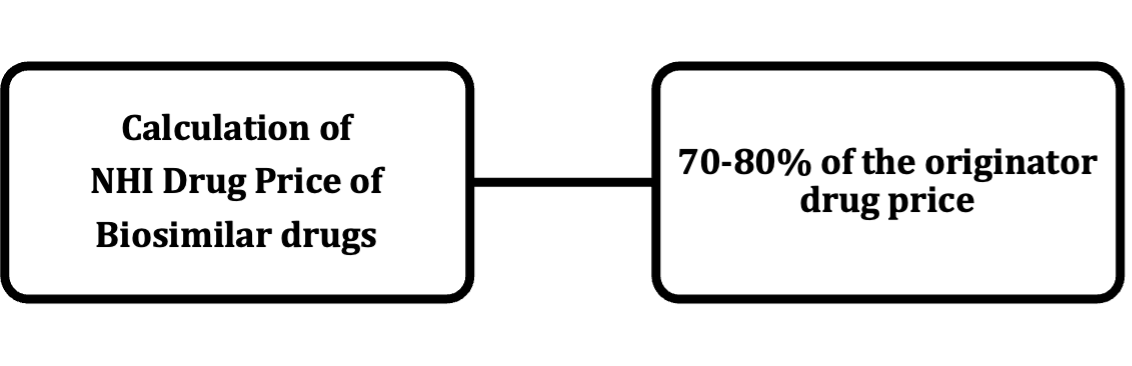
In principle, the NHI Drug Price of a biosimilar drug is 70% of the price of the originator drug calculated by the comparable drug method. An additional price will be added, up to 10%, depending on the depth of clinical trials that were conducted for the biosimilar drug.
[Overview of NHI Drug Price Standards of generic drugs and biosimilar drugs]
3. What are the reimbursement approval processes and principles for originator drugs?
The reimbursement approval processes and principles for originator drugs are as described in Question II. 3.
4. What are the reimbursement approval processes and principles for generics and biosimilar drugs?
The reimbursement approval processes and principles for generics and biosimilar drugs are the same as those for originator drugs, which are described in Question II. 3.
Also from this Market Access & Health Technology Assessment
2. Expenditure Control and Cost-Containment Policies: Japan
- Please describe the main cost containment policies in place in your country and their fundamental principles
a. Pricing and impact of generic/biosimilar approval
(i) Pricing
Generics and biosimilars are priced lower than their branded equivalents, reflecting lower R&D costs.
When a generic drug is first listed on the NHI Drug Price List, its price is set at 50% of the branded drug’s price (which is calculated by the comparable drug method, and hereinafter the same). If it is an oral drug and more than seven generics for the same drug are listed at once, their price drops to 40% of the branded drug’s price.
Biosimilars are priced at 70% of the original branded drug’s price when they are first listed on the NHI Drug Price List. If it is an oral drug and more than ten generics for the same drug are listed at once, their price drops to 60% of the branded drug’s price. A certain percentage, 10% at maximum, may be added according to the depth of clinical trial that was conducted for such biosimilar. This higher price is because biosimilars are more complex and expensive to produce than generics.
(ii) Approval
Generics must be verified to be therapeutically equivalent to the original drugs in a bioequivalence study. The information and data required for generic drug approval are limited compared to original drugs.
On the other hand, it is difficult to demonstrate the identity of the active ingredient in a biosimilar due to its large molecular weight and complex structure. Therefore, in order to obtain marketing authorization for a biosimilar, it is necessary to demonstrate equivalence in terms of quality, safety and efficacy with the preceding biopharmaceutical by conducting clinical trials and other studies.
b. Clawback/Payback/Discounts/Rebates
It is common industry practice for pharmaceutical companies to provide rebates and allowances to pharmaceutical wholesalers.
It also is common for pharmaceutical wholesalers to sell and deliver drugs to medical institutions and pharmacies before they agree on purchase prices. This practice undermines the credibility of the drug price surveys based on which the MHLW revises NHI Drug Prices.
The MHLW is concerned about these practices and discusses how to improve the situation at the Roundtable on Improvement of Distribution of Medical Drugs.
c. Existence of Price/Volume agreements in the frame of public tendering
N/A
d. Existence of price freezes and cuts
The NHI Drug Prices generally are reduced in annual NHI Drug Price Revisions.
In addition, if the market sales of a drug significantly exceed initial forecasts, the NHI Drug Prices will be reduced (“Market Expansion Re-pricing (Shijo Kakudai Sai-Santei)”). If Market Expansion Re-pricing is applied to a certain drug, the NHI Drug Prices of other drugs with similar indications and pharmacological actions may be reduced also.
The NHI Drug Prices of drugs that appear on the Essential Drug List (“EDL”) are maintained in accordance with the rules for Essential Drugs, which are explained in the response to Question XI-2.-c. Other unprofitable drugs due to their low NHI Drug Prices are given special consideration.
e. Post-launch monitoring of prescriptions/sales
The MHLW surveys the sales prices and volumes of drugs from pharmaceutical wholesalers to medical institutions and pharmacies by inquiries to those parties. The result of the survey is used as the base of the annual NHI Drug Price Revision.
f. Existence of Generic Substitution Policies
The Japanese government aimed to have 80% in volume terms and 65% in value terms of all prescribed drugs be generics by March 31, 2030, while maintaining a stable supply of generics. For biosimilars, the goal is that the number of biopharmaceuticals whose 80% of the volume is replaced by biosimilars reaches 60% or more of the total active ingredients of biopharmaceuticals by March 31, 2029.
g. At prescriber level
Doctors receive additional medical fees if they choose to prescribe a generic drug instead of a brand-name drug.
h. At retail level
Pharmacies receive extra fees when they dispense generic drugs. This extra payment serves as an incentive for pharmacies to promote and dispense generic drugs.
- Are there any other policies in place aiming at cost control via incentive programs targeting the different actors (pharma companies, wholesalers, retailers, prescribers etc)?
Individuals may enjoy tax breaks under the Self-Medication Taxation program. If (i) the cost of purchasing OTC drugs exceeds JPY 12,000 and (ii) the relevant person takes measures to maintain his or her health, including having Specific Health Check-Ups, vaccinations, regular health check-ups, medical examinations, and cancer screenings, the individual is able to receive a tax break for the cost of purchasing OTC drugs in excess of JPY 12,000, in an amount up to JPY 88,000. This incentivizes individuals to use OTC drugs as substitutes for prescription drugs and reduces national medical expenses.
Also from this Market Access & Health Technology Assessment
3. Public Procurement and Tendering: Japan
- Which are the main actors involved in public procurement and tendering?
Please see the response to Question II.-7.
- What are the main characteristics of the public procurement and tendering system?
Japan has established voluntary measures, such as tendering procedures based on stricter standards than those in the WTO-GPA, for certain products and services. With regard to healthcare, the government established the Measures on Procurement of Medical Technology Products and Services in the Japanese Public Sector.
Among other things, the voluntary measures require: (i) a longer prior notice period for public tenders (i.e., 50 days from the announcement of public tender to a deadline for bidding), and (ii) more strict requirements for setting specifications and a more strict process for public procurement, to ensure fair competition.
Also from this Market Access & Health Technology Assessment
4. Managed Entry Agreements: Japan
- Are there any Managed entry agreements in place in your country? (If so, please list them)
No.
For each Individual Managed entry Agreement
- Describe the fundamentals of the Managed entry Agreement, its rationale and the process for implementing it.
N/A
- When should this Managed Entry Agreement be considered?
N/A
- Which are the specific requirements to implement the Managed Entry Agreement.
N/A
- What is the potential impact on the product uptake?
N/A
- What are the potential challenges associated with this Managed Entry Agreement?
N/A
Also from this Market Access & Health Technology Assessment
5. Data Requirements: Japan
- In addition to the clinical data obtained through clinical studies please list the data required for
a. Market approval
In general, an applicant for marketing authorization for a new drug is required to submit the information and data below.
| Documents | |
| a. Origin or background of discovery, condition of use in foreign countries | 1 Origin or background of discovery
2 Conditions of use overseas 3 Special characteristics, comparisons with other drugs, etc. |
| b. Manufacturing methods, specification and test methods | 1 Chemical/physical characteristics and structure property
2 Manufacturing methods 3 Specification and test methods |
| c. Stability | 1 Long-term storage tests
2 Tests under severe conditions 3 Accelerated tests |
| d. Pharmacological action | 1 Tests to support efficacy
2 Secondary pharmacology, safety pharmacology 3 Other pharmacology |
| e. Absorption, distribution, metabolism, and excretion | 1 Absorption
2 Distribution 3 Metabolism 4 Excretion 5 Bioequivalency 6 Other pharmacokinetics |
| f. Acute/sub-acute/chronic toxicity, teratogenicity, and other types of toxicity | 1 Single dose toxicity
2 Repeated dose toxicity 3 Genotoxicity 4 Carcinogenicity 5 Reproductive toxicity 6 Local irritation 7 Other toxicity |
| g. Clinical trials | 1 Results of clinical trials |
| h. Package inserts | 1 Points to consider of package inserts |
b. Pricing Decisions
In general, an applicant for listing a new drug on the NHI Drug Price List is required to submit the information and data below.
| Documents |
| 1 The NHI Drug Price Request form
2 The NHI Drug Price Calculation form 3 Copy of foreign price list 4 Copy of the New Drug Examination Report 5 Either (i) Common Technical Document, Part 1, (5) history of origin or discovery and development, (6) data on usage in foreign countries, (7) list of similar products, and (9) documents pertaining to generic name, or (ii) the origin or discovery of the drug and its status of use in foreign countries in the summary of documents submitted to the First Drug Subcommittee or the Second Drug Subcommittee of the Pharmaceutical Affairs and Food Sanitation Council, whichever is applicable. 6 Draft package insert, draft indications, dosage and administration, draft precautions and basis for precautions, draft precautionary statements, draft drug risk management plan 7 Either (i) Common Technical Document, Part 1 (excluding (2) to (4)) and Part 2, (2) introduction, (3) overview of quality, (4) overview of non-clinical, (5) overview of clinical or (ii) copy of the summary of documents submitted to the First Drug Subcommittee or the Second Drug subcommittee of the Pharmaceutical Affairs and Food Sanitation Council. 8 Copy of a marketing authorization |
c. Reimbursement Decisions
In general, medical institutions or pharmacies that are applying for reimbursement are required to submit the information and data below to the Claims Review and Reimbursement Agencies.
| Documents |
| 1 Medical fee request form
2 Medical fee schedule (“Receipt”) |
Also from this Market Access & Health Technology Assessment
6. HTA Dossiers: Japan
- Have local authorities published recommendations surrounding value assessment dossiers? (If yes please add link)
Marketing authorization holders of products designated as subject to the Cost-Effectiveness Evaluation System are required to conduct an analysis of the cost-effectiveness evaluation of the designated products and submit a report describing the analysis data. With regard to how to prepare such reports, C2H has prepared the “Forms and Guidance for Describing Analysis Results for Cost-Effectiveness Evaluation of Drugs and Medical Devices”. The link to this guidance is as follows: https://c2h.niph.go.jp/tools/system/Reporting_Format_Jap_202404.pdf (Japanese version only)
In addition, the guidance states that target products shall be analyzed based on the “Guidelines for Analysis of Cost-Effectiveness Evaluation at the Central Social Insurance Medical Council” and the analysis framework determined by the Specialized Body for Cost-Effectiveness Evaluation. The link to this guideline is as follows. In case of any discrepancy between the English version and the Japanese version, the Japanese version shall take precedence.
[Japanese version]
https://c2h.niph.go.jp/tools/guideline/guideline_ja_2024.pdf
[English version]
https://c2h.niph.go.jp/tools/guideline/guideline_en_2024.pdf
Further, past results of the Cost-Effectiveness Evaluation System can be found for reference at the following link: https://c2h.niph.go.jp/results/item.html
- Have local Authorities published guidelines surrounding value assessment dossiers? (If yes please add link)
As described in 1. above.
- Have local authorities published official guidelines surrounding the submission of value assessment dossiers? (If yes please add a link)
As described in 1. above.
- Describe the overall process of preparing and submitting a HTA dossier in your country.
Marketing authorization holders must submit data on the analysis results, including the analysis method, conditions, and ICER, in principle within 9 months from the date Chuikyo designates the target product. In the first 3 to 6 months of the 9-month period, pre-analysis consultations are held between the marketing authorization holder and C2H. Specifically, (1) the marketing authorization holder submits a draft framework for analysis, (2) consultations and discussions are conducted based on the submitted draft framework, and (3) the contents of the consultations are recorded in writing. In the subsequent 3-6 months, the marketing authorization holder analyzes the HTA of the target product based on the analysis framework discussed with C2H and submits the analysis to the Specialized Body for Cost-Effectiveness Evaluation. During this period, the marketing authorization holder will hold necessary discussions with C2H. The subsequent process is as described in V.1 above.
- Describe the overall content of the HTA dossier in your country.
According to the “Forms and Guidance for Describing Analysis Results for Cost-Effectiveness Evaluation of Drugs and Medical Devices” (see 1 above), the items of documents to be submitted by marketing authorization holders of products designated as items subject to the Cost-Effectiveness Evaluation System are as follows.
- Nature of the target drug or medical device
Name, reimbursement price, mechanism of therapeutic effect, target disease, method of use, position of the product in the treatment of the target disease, major adverse events, results of evaluation by medical technology evaluation organizations in other countries, etc.
- Establishment of analytical conditions for cost-effectiveness analysis
Analysis population, comparator technology, analysis position and cost range, effectiveness index, analysis period, discount rate, etc.
- Additional usefulness
Research questions and results of the systematic review, details of the meta-analysis, results of indirect comparisons and network meta-analysis, evaluation of the presence or absence of additional usefulness, etc.
- Details of analysis method
Methodology used to calculate cost-effectiveness, assumptions used in the model and definition of health status, details of parameters used in the analysis (effectiveness, safety, quality of life values, costs, etc.)
- Analysis results
Analysis results, sensitivity analysis, review of validity of analysis results, interpretation of analysis results, analysis including public costs for caregiving and productivity losses, etc.
- Which are the questions to focus on when preparing a HTA dossier in your country?
It would be advisable for the marketing authorization holder of a target product to consider the following items when preparing a report analyzing cost-effectiveness.
- Unresolved needs based on patient burden due to the disease (what needs the product is trying to solve)
- Clinical position of the product in the treatment of the disease (how the product can contribute to the unmet needs described above, based on efficacy and safety outcome data obtained in clinical trials and actual clinical practice).
- Socio-economic impact of the product (clarify the various values of the product in the treatment of the disease, such as improvement of patients’ health and daily life, reduction of disease care, and effective utilization of medical resources through improved treatment delivery methods, as well as financial impact).
- The evidence should be of high quality, transparent, comprehensive, and balanced from a scientific perspective.
- Which are the other strategic considerations to take into account when preparing a HTA dossier in your country?
None in particular.
Also from this Market Access & Health Technology Assessment
7. HTA Decision Analysis Framework: Japan
- Which are the health technology assessment (HTA) evaluation bodies and their responsibilities in your country?
In Japan, it is important whether a drug or medical device is covered by the NHI (i.e., whether it is listed on the NHI Price List). If it is, the product is eligible for NHI reimbursement. (Note that in Japan, only medical devices belonging to the category of “Specified Insured Medical Materials” can be listed on the NHI Price List.) In Japan, HTA is not taken into account in determining whether to list a product on the NHI Price List or not. However, the NHI Price of listed drugs and medical devices (including regenerative medical products treated in the same way as drugs or medical devices) is adjusted by HTA. This system is called the Cost-Effectiveness Evaluation System. (Note that “NHI Price” refers to the price of a drug or medical device that is used as a standard when reimbursement is made to medical institutions and pharmacies by insurers (such as health insurance associations) based on the NHI system.)
The Cost-Effectiveness Evaluation System will follow the following process.
- The Central Social Insurance Medical Council (“Chuikyo”) selects the target products.
- The marketing authorization holder of the target product conducts a cost-effectiveness analysis and submits a report containing the analysis data to the Specialized Body for Cost-Effectiveness Evaluation (a body set up under Chuikyo).
- The Center for Outcomes Research and Economic Evaluation for Health (“C2H”) reviews the analysis data submitted by the marketing authorization holder of the target product and reports the result to the Specialized Body for Cost-Effectiveness Evaluation. In practice, C2H has a public analysis team (designated by C2H) that conducts the review.
- The Specialized Body for Cost-Effectiveness Evaluation prepares a draft of the cost-effectiveness evaluation and reports it to Chuikyo’s General Assembly.
- Upon receiving the above report, Chuikyo’s General Assembly adjusts the NHI Price of the target products.
As described above, C2H (including a public analysis team established under C2H) and Chuikyo (including the Specialized Body for Cost-Effectiveness Evaluation established under Chuikyo) play an important role in HTA in Japan.
- Do regulators require HTA studies in your country?
The Cost-Effectiveness Evaluation System is not implemented for all drugs and medical devices, but only for those designated by Chuikyo. On designation, Chuikyo takes into consideration the degree of innovation of drugs and medical devices and the impact on healthcare finances.
Specific selection criteria are described in the following link: https://www.mhlw.go.jp/content/000497467.pdf (Japanese only). For example, in the case of a drug that is listed after the establishment of the Cost-Effectiveness Evaluation System and whose NHI Drug Price is determined in accordance with the Similar Drug Price Method (*1), it meets selection criteria if the Usefulness Related Addition (*2) was made and it falls under one of the following:
・Its peak market size (forecast): 10 billion yen or more;
・Its peak market size (forecast): 5 billion yen or more but less than 10 billion yen; or
・Judged by Chuikyo’s General Assembly as necessary to be subject to the Cost-Effectiveness Evaluation System for reasons such as its significantly high unit price.
*1 Similar Drug Price Method: Method to determine the NHI Drug Price based on existing products that are similar in terms of efficacy, target disease, route of administration, etc.
*2 Usefulness Related Addition: (i) Usefulness Addition, addition to NHI Drug Price that is made when a new product has a higher usefulness than existing products, and (ii) Breakthrough Addition, addition to NHI Drug Price that is made when a new product has a new clinically useful mechanism of action or when it is objectively shown to have higher efficacy or safety compared to existing similar products. Usefulness Related Addition can be made only if the NHI Drug Price was originally determined in accordance with the Similar Drug Price Method.
The following items are excluded from the Cost-Effectiveness Evaluation System. This is because the number of eligible patients is small and the original unit price tends to be high.
・Items used only for rare diseases for which there are no adequate treatment methods (designated intractable diseases, haemophilia, and HIV infection)
・Items used only for children
- However, even for items that fall under the above, items with a market size of 35 billion yen or more or items with a significantly high unit price may be subject to the Cost-Effectiveness Evaluation System at the discretion of the Chuikyo’s General Assembly. Furthermore, if some of the indications include rare diseases or pediatric diseases but the indication is not limited to rare diseases or pediatric diseases, the product will not be excluded from the Cost Effectiveness Evaluation system. However, in this case, consideration will be given in the comprehensive evaluation and price adjustment (see 6.(4) below).
- Do payers require HTA studies in your country?
Payers such as insurers (including health insurance associations) are not involved in the Cost-Effectiveness Evaluation System.
- How are HTA assessments translated into pricing conditions in your country?
Although HTA is not implemented at the time of listing of drugs or medical devices on the NHI Price List, the NHI Prices of drugs and medical devices, if designated, can be revised based on the Cost-Effectiveness Evaluation System as described in 1. above. The revision of NHI Prices based on the Cost-Effectiveness Evaluation System is carried out four times a year. The specific method of NHI Price revision is described in 6. below.
- How are HTA assessments translated into reimbursement conditions in your country?
As stated in 4. above, HTA is not conducted at the time of listing of drugs and medical devices on the NHI Price List. This is because if we require superior cost-effectiveness as a condition for reimbursement, it would impede patient access to products and create a drug lag due to the time required to list the product on the NHI Price List. However, the Ministry of Finance insists that the Cost-Effectiveness Evaluation System should be used to determine whether a product is covered by NHI. Such a framework is still under consideration.
- Which are the evaluation criteria, processes or models and analyses framework used for HTA in your country?
(1) Cost-Effectiveness Evaluation Methodology
Cost-effectiveness evaluation is performed with ICER (QALY).
(2) Portion subject to price adjustment
Price adjustment will be made to the following portions of the NHI Prices of drugs or medical devices.
(a) Items whose NHI Price is calculated based on the Similar Drug Price Method
The portion of Usefulness Related Addition shall be subject to price adjustment.
(b) Items for which the NHI Price is calculated based on the Cost Calculation Method
The portion subject to price adjustment depends on whether the target product is a drug or medical device, whether the disclosure level (*) exceeds 50%, and when the NHI Price was listed. For more information, please refer to the following link: https://www.mhlw.go.jp/content/000497467.pdf (Japanese only).
* The “disclosure level” is the percentage of costs (e.g., manufacturing costs, selling expenses, and general and administrative expenses) that were disclosed to the government organization when the company applied for listing on the NHI Price based on the Cost Calculation Method.
(3) Price adjustment rate
The price adjustment rate is set forth as follows.
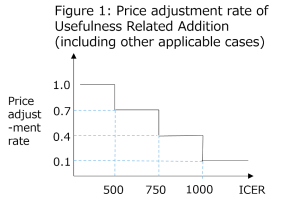
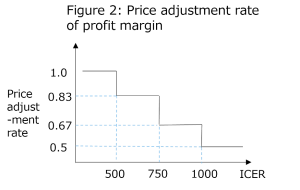
The price adjustment will be made using the above price adjustment rate according to the following formula.
In case of price adjustment of Usefulness Related Addition (including other applicable cases): The amount of Usefulness Related Addition before price adjustment – the amount of Usefulness Related Addition × (1 – price adjustment rate)
In case of price adjustment of profit margin: The amount of profit margin before price adjustment – the amount of profit margin × (1 – price adjustment rate)
(4) Consideration in Comprehensive Evaluation
For the following items, the price adjustment rate will be relaxed as follows.
・Items that include rare diseases for which there are no sufficient treatment methods (designated intractable diseases, haemophilia, and HIV infection) as part of their indications
・Items that include pediatric diseases as part of their indication and for which pediatric dosage and administration are approved
・Oncology drugs
(5) Lowering Ceiling
The following lowering ceilings (beyond which no further reductions will be made by the Cost-Effectiveness Evaluation System) have been established. This is to ensure a stable supply of necessary drugs and medical devices for patients.
(i) Items whose percentage of Usefulness Related Addition in the NHI Price (before such addition was made) is 25% or less: The lowering ceiling is the price reduced by 10% from the NHI Price before adjustment.
(ii) Items whose percentage of Usefulness Related Addition in the NHI Price (before such addition was made) exceeds 25% and is less than 100%: The lowering ceiling is the price reduced by the rate calculated based on the following formula from the NHI Price before adjustment.
{10 + (Usefulness Related Addition rate – 25)/15}%
(iii) Items whose percentage of Usefulness Related Addition in the NHI Price (before such addition was made) is 100% or more: The lowering ceiling is the price reduced by 15% from the NHI Price before adjustment.
(6) Price increase
The NHI Price will be increased for products that fulfil the following conditions, as it is considered preferable to utilize these products from a cost-effectiveness standpoint.
- Where the ICER is not calculable (i.e., where the effect of the target product is greater than or equal to that of the comparator product (*) and costs are reduced)
An amount equivalent to 50% of the potion subject to price adjustment described in (2) above (provided, excluding profit margin) shall be increased if the following requirements are met:
- the item is shown in clinical trials to be more effective than or equivalent to the comparator product (to be noted that an item that is more effective and less expensive than the comparator product is specifically referred to as “dominant”); and
- the item is completely different from the comparator product, or its improvement is beyond the scope of general improvement (e.g., having a different basic structure or principle of action).
However, the amount of the increase shall be subject to the following limitations:
- The amount of increase shall not exceed 10% of the total NHI Price: and
- The cost saving per patient compared to the comparator item after the price adjustment does not exceed the amount equal to one-half of that of before the price adjustment.
- Where the ICER is calculable (i.e., the effect of the target product is greater than that of the comparator product) and the ICER is less than 2 million JPY/QALY
An amount equivalent to 25% of the portion subject to price adjustment described in (2) above (excluding profit margin) shall be increased if the following requirements are met:
- the item is shown in clinical trials (subject to additional conditions regarding the scope of the clinical trials) to be more effective than the comparator product; and
- the item is completely different from the comparator product, or its improvement is beyond the scope of general improvement (e.g., having a different basic structure or principle of action).
However, the amount of the increase shall be subject to the following limitations:
- The amount of increase shall not exceed 5% of the total NHI Price: and
- ICER calculated based on the price after price adjustment does not exceed 2 million JPY/QALY.
- The term “comparator item” refers to an existing product that is widely used in clinical practice and is expected to be substituted by the target product.
- What is the methodology used in your country for HTA assessment?
As discussed above, cost-effectiveness is calculated mainly by the incremental cost-effectiveness ratio (ICER) method.
- Which are the other decisions impacted by the assessed outcome in your country?
The supply of drugs and medical devices in Japan is dependent on the NHI system, and eligibility for insurance coverage is a prerequisite for their distribution in the market. The Cost-Effectiveness Evaluation System has a significant impact on the business of drug or medical device companies and their distributors because it influences drug prices and reimbursement prices. During the development phase of a new drug or medical device, it becomes necessary to consider how cost-effective the product will be in the future compared to existing similar products.
- Does your HTA review or inquire other international HTAs during the assessment process? If so, which ones are the usual partners?
When C2H (or more precisely, a public analysis team designated by C2H) reviews the analysis data submitted by marketing authorization holders, it refers to the results of evaluations conducted by medical technology evaluation organizations in other countries. According to the “Guidelines for Analysis of Cost-Effectiveness Evaluation at the Central Social Insurance Medical Council” (see VI.1) prepared by C2H, when a marketing authorization holder makes a report describing analytical data, it is required to indicate, as “analytical methods,” existing cost-effectiveness analyses of the technology and evaluation results published by major foreign medical technology evaluation organizations in major foreign countries, if any. Specifically, according to the “Forms and Guidance for Describing Analysis Results for Cost-Effectiveness Evaluation of Drugs and Medical Devices” (see VI.1) prepared by C2H, a marketing authorization holder is required to include in its report at least the evaluation results of the following medical technology evaluation organizations; if the relevant evaluation results do not exist in relation to all or some of the organizations, this must also be stated in the report. The inclusion of evaluation results at other organizations is optional.
- NICE (UK-England/Wales): Technology appraisal, Medical technologies guidance (medical devices)
- SMC (UK/Scotland): SMC advise
- HAS (France): SMR/ASMR, efficiency assessment
- IQWiG (Germany): Additional efficacy assessment by AMNOG (drugs)
- CADHT (Canada): Common Drug Review or pan-Canadian Oncology Drug Review (drugs), other CADDTH reports (medical devices)
- Health Quality Ontario (Ontario, Canada): Medical devices
- PBAC (Australia): Drugs
- MSAC (Australia): Medical Devices
- ICER (USA)
Also from this Market Access & Health Technology Assessment
8. Price Control and Reference Pricing Systems: Japan
1.Price Control
- How does price control at ex-factory prices work in your country?
There is no control by the government on the sales price of drugs from a market authorization holder to a pharmaceutical wholesaler regardless of whether it is a prescription drug or an over-the-counter drug.
- How does price control at the wholesale level work in your country?
There is no control by the government on the sales price of drugs from a pharmaceutical wholesaler to a medical institution or pharmacy, regardless of whether it is a prescription drug or an over-the-counter drug.
Therefore, a pharmaceutical wholesaler is supposed to determine the sales price to a medical institution or pharmacy by adding a certain margin to the purchase price. However, in practice, as a result of price negotiations, the sales price from a pharmaceutical wholesaler to a medical institution or pharmacy is sometimes set lower than the purchase price, which results in a loss of the pharmaceutical wholesaler. In such a case, it is typical that the pharmaceutical wholesaler receives various rebate allowances from the marketing authorization holder to make up such loss and obtain a certain margin.
In addition, medical institutions and pharmacies earn marginal profits by purchasing drugs from pharmaceutical wholesalers at a price lower than the NHI Drug Price which will be reimbursed to them.
[Pricing mechanism at the wholesale level]
| NHI Drug Price
(The price from medical institutions to patients) |
| Sales price from pharmaceutical wholesalers to medical institutions |
| Sales price
from marketing authorization holders to pharmaceutical wholesalers |
| Profit of medical institutions |
| Rebate allowancess from marketing authorization holders to pharmaceutical wholesalers |
| Profit of pharmaceutical wholesalers |
- How does price control at the retail pharmacy level work in your country?
The prices of prescription drugs sold to patients at insured pharmacies are determined based on the NHI Drug Prices. Patients pay 30% (or other applicable self-pay rate) of the NHI Drug Price. The details of calculation methods of the NHI Drug Price are discussed in Question I. 2. A. above. Over-the-counter drugs are not subject to the NHI Drug Price and can be sold to patients at any price.
In addition, as mentioned in Question IV. 1. 2. above, the sales price from a pharmaceutical wholesaler to a pharmacy can be also freely determined, regardless of whether it is a prescription drug or an over-the-counter drug.
2.External Reference Pricing (ERP)
- Is there a system of external reference pricing (ERP) in place in your country?
There are rules for adjusting the NHI Drug Prices of originator drugs by referring to external reference pricing (ERP) (i.e., foreign average price adjustment).
- When and/or how often is ERP activated?
After calculating the NHI Drug Price of an originator drug either by the comparable drug method or the cost accounting method (as detailed in Question III. 3. above), the foreign average price adjustment will be made if there is a gap between the calculated NHI Drug Price and the average sales price of the drug in other countries.
- What is the legal framework of the ERP in place in your country?
The foreign average price adjustment is provided under the Health Insurance Act and related regulations as a part of the NHI Drug Price Standards.
- What is the composition of the country basket?
The reference price used for the foreign average price adjustment is calculated based on the sales price of the same drug in the United States, the United Kingdom, Germany, and France.
- Describe the price calculation and selection for reference products.
The reference price used for the foreign average price adjustment is the average price of a drug, that has the same composition and formulation category and similar specifications and actual use, listed on the price list in the United States, the United Kingdom, Germany, and France (for the United States, prices listed on the Medicare or Medicaid price list are used).
If (i) there are two or more countries where the subject drug is listed on the price list and (ii) the highest price is more than 2.5 times the lowest price, the highest price should be excluded in calculating the average.
If (i) there are three or more countries where the subject drug is listed on the price list and (ii) the highest price is more than two times of the average price of other countries, the highest price should be replaced with the average price of other countries (and then the average price should be calculated again).
- How often does the price need to be updated?
ERP (i.e., listed price in other countries) as well as exchange rates as of the date of listing of the originator drug on the NHI Drug Price List are used.
- How do the “price List”/catalogues from references countries work in your country?
A foreign average price adjustment is made in the following cases.
(1) If the price calculated either by the comparable drug price method or the cost accounting method exceeds 125% of the foreign average price, the following downward adjustment is made.
(1/3 x calculated price / foreign average price + 5/6) x foreign average price
(2) If the price calculated either by the comparable drug method or the cost accounting method is less than 75% of the foreign average price, the following upward adjustment is made.
(1/3 x calculated price / foreign average price + 1/2) x foreign average price
3.Internal Reference Pricing (IRP
- Is there an internal reference pricing (IRP) system in your country?
As discussed in Question III. 3. above, if there is a similar drug, the NHI Drug Price of an originator drug is calculated by referring to the NHI Drug Price of such a similar drug (i.e., the comparable drug method).
- What is the legal framework of the IRP in place in your country?
IRP (i.e., the comparable drug method) is provided under the Health Insurance Act and related regulations as a part of the NHI Drug Price Standards.
- When and/or how often is IRP activated?
As long as there is a similar drug, the comparable drug method is used for calculating the NHI Drug Price of an originator drug.
Also from this Market Access & Health Technology Assessment
9. Healthcare Actors and Payers: Japan
- Which are the administrations, bodies and institutions in charge of public health in your country and what are their respective responsibilities?
The MHLW, the PMDA, and the Central Social Insurance Medical Council are responsible for public health. Their respective roles are explained in the response to Question I.-1.
2. Which are the administrations, bodies and institutions in charge of drug approvals in your country and what are their respective responsibilities?
The MHLW is responsible for approving marketing of drugs in Japan. Pharmaceutical and Food Sanitation Council serves as an advisory body to the MHLW, and reviews drug marketing authorization applications.
The PMDA is responsible for reviewing applications for marketing authorization for drugs.
Some types of drugs, including over-the-counter (“OTC”) drugs, such as cold drugs, pain relievers, cough drugs and gastrointestinal drugs, are approved by the prefectural government instead of the MHLW.
3. Which are the administrations, bodies and institutions that qualify as “payers” in your country and what are their respective responsibilities?
When a patient receives medical services and purchases drugs, the patient pays part of the medical costs (10%, 20% or 30% depending on age and other conditions) at a medical institution/pharmacy and the insurer (e.g. health insurance association) pays the rest to the insured medical institution/pharmacy. The Claims Review and Reimbursement Agencies check the receipts submitted by the medical institution/pharmacy for correct billing and then pay the reimbursement to the insured medical institution/pharmacy.
The relevant parties and reimbursement process are as follows:

4. Which are the administrations, bodies and institutions in charge of pricing decisions in your country and what are their respective responsibilities?
The MHLW and the Central Social Insurance Medical Council are responsible for NHI Drug Pricing decisions.
NHI Drug Prices are regulated under the Health Insurance Act. After obtaining marketing authorization, a pharmaceutical company submits a request to the MHLW to list the NHI Drug Price of its newly approved product. After the consultation with the Central Social Medical Council, the MHLW determines the NHI Drug Price of such product and lists it on the NHI Drug Price Standard List. The methods of calculation of NHI Drug Price are disclosed and revised every year by the MHLW.
The prices of medical devices are included in Medical Fees; except for certain types of medical devices that are provided their own NHI Prices separately from Medical Fees (e.g., artificial cardiac valve, pacemaker, PTCA catheter, “Special Treatment Materials”). Those prices also are regulated by the MHLW under the Health Insurance Act. The prices of Medical Fees and Special Treatment Materials are determined and announced by the MHLW after consultation with the Central Social Insurance Medical Council.
5. Which are the administrations, bodies and institutions in charge of reimbursement decisions in your countries and what are their respective responsibilities?
As discussed in II-3 above, the Claims Review and Reimbursement Agencies and Insurers (e.g., corporate health insurance unions) are responsible for reimbursement decisions. The Claims Review and Reimbursement Agencies review medical claims from medical institutions and pharmacies to verify the accuracy of claims. After verification by the Claims Review and Reimbursement Agencies, insurers, through the Claims Review and Reimbursement Agencies, reimburse medical institutions and pharmacies for medical expenses based on the Medical Fee Point List and NHI Drug Price Standard List.
6. Which are the administrations, bodies and institutions in charge of Health Technology Assessment in your countries and what are their respective responsibilities?
Please refer to Question V.1.
7. Which are the administrations, bodies and institutions in charge of public procurement and tendering in your country and what are their respective responsibilities
Public procurement is governed by the Ministry of Finance ( “MOF”) and the MHLW. The MOF is responsible for the overall public procurement policy in Japan, including the establishment of rules and guidelines that apply across all sectors. The MHLW is responsible for public procurement in the healthcare sector specifically, in line with the broader rules established by the MOF.
The MHLW, national/public hospitals, and national/public research centers designated as central/local government organizations, as well as other organizations referenced in the World Trade Organization’s Agreement on Government Procurement (“WTO-GPA”) are subject to public procurement.
8. What are the other actors of significance with regards to market access in your country and what are their respective responsibilities?
N/A
Also from this Market Access & Health Technology Assessment
10. Healthcare System and Funding: Japan
1.Please make a general introduction to the public health sector in your country and its organization
(a) The overview of the public health sector
In Japan, the universal health insurance system allows citizens to receive medical services anytime, anywhere. The system is run by insurance premiums and other funds collected from the citizens. When patients receive medical services, they pay a certain portion (10%, 20% or 30% depending on age and other conditions) of the medical fees and drug price to the medical institution/pharmacy and the rest is paid by the health insurer (e.g. health insurance association and other health insurer). The prices of medical services (including medical devices) and drugs covered by insurance are determined by the government.
(b) The organization of the public health sector
The Ministry of Health, Labour and Welfare (the “MHLW”) is the government body that oversees the healthcare system and establishes policies.
The Pharmaceuticals and Medical Devices Agency (“PMDA”) is another important organization, which is responsible for reviewing applications for pharmaceuticals and medical devices, as well as overseeing post-marketing safety measures, and relief services for adverse health events.
The Central Social Insurance Medical Council is an advisory body to the MHLW, which is comprised of three groups of representatives from the medical fee payors, the medical services providers, and the parties representing the public interest such as academia and patients. The Central Social Insurance Medical Council holds various discussions throughout the year, mainly concerning medical fees and drug prices under the National Health Insurance (“NHI”).
The Main Budget Office, an internal department of the Ministry of Finance, is responsible for the national budget related to social security and is one of the important players in public health.
2. Please provide any infographics including
The actors involved in the market access process (market authorization, pricing decisions, reimbursement decisions)
The information and data required
The process and flow
The actors involved in the market access process of new drugs, and the flow is shown in the chart below (items a and c above).

In general, an applicant for marketing authorization for new drugs is required to submit the following information and data (item b above):
- Origin or background of discovery, condition of use in foreign countries
- Manufacturing methods, specifications, and test methods
- Stability
- Pharmacological action
- Absorption, distribution, metabolization, and excretion
- Acute/sub-acute/chronic toxicity, teratogenicity, and other types of toxicity
- Clinical trials
- Package inserts
For more details, please refer to Question VII.1.a.
In order to apply for listing of new drugs on the NHI Drug Price Standard List, it is necessary to provide certain information and data, such as the NHI Drug Price request, the NHI Drug Price calculation form, and information regarding price and use in foreign countries.
For more details, please refer to Question VII.1.b.





